For many centuries, it has been observed that gold has been in use in many applications, whether it remains for decorating purposes, functional benefits, or industrial applications. As the world grew more connected, the benefits of gold plating have been utilized faster than ever before. An example of this is the swift and stable signal transmission using highly reliable contacts and connectors of gold. Meanwhile, several manufacturing companies come into the field to deliver gold plating services across various medical, defense, and telecommunication industries.
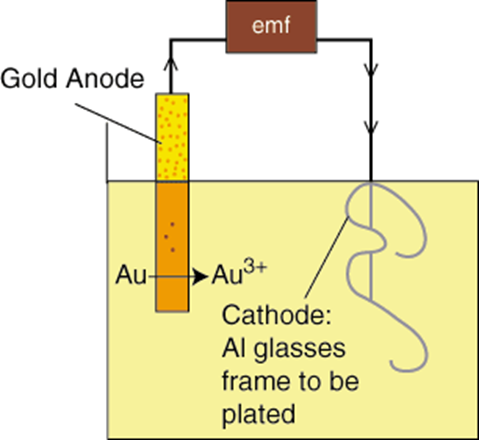
A thin layer of gold is deposited onto the surface of another metal using an electrochemical process, which is known as gold plating. The metal is gold plated by putting it into a gold bath solution and using the electric current to draw the positively charged gold ions to stick to the metal piece, which is negatively charged. Besides that, gold plating is also commonly done to brighten the appearance of old jewelry whose color has faded. Still, it likewise has many applications in different industries, as mentioned above, such as in the medical department and outer space.
Gold Plating Types
Commonly gold plating is broken down into three primary categories: hard gold plating, soft gold plating, and duplex gold plating (which remains a blend of both). Along the same lines, these three types of gold plating services have been described below:
Hard Gold Plating
In hard gold plating, to improve the hardness and wear properties of the gold, we add a small amount of an alloying element or compound to the gold. Nickel and cobalt are the metals, which are commonly used for this task, but sometimes, iron and arsenic can also be utilized. Gold appears brighter in hard gold deposits due to it having a more refined grain structure. The applications in which sliding contact or constant engagement occurs take benefit from hard gold plating as it has increased hardness and allows for long and continuous use without breaking down.
Soft Gold Plating
In soft gold plating, contrary to hard gold plating, where a small amount of element was added, we use incredibly pure gold having a purity of 99.9% or more. A soft gold deposit attains the maximum hardness of 90 Knoop, which is lower than that of hard gold, and its grain structure is also less refined compared to hard gold. Since no other elements are present in soft gold to oxidize, soft gold is considered excellent for high-temperature applications and wire bonding. Also, soft gold is ideal for low-load static contact designs such as lapping connections. Due to its high purity and biocompatibility of the finishing, it is additionally preferred for medical applications.
Duplex Gold Plating
In duplex gold plating, both hard and soft gold deposits are used to create a finish, which has the properties of both soft gold plating and hard gold plating. Let’s say to improve the wear properties of hard gold. In that case, we can coat a layer of soft gold over it, which highly resists corrosion while enhancing the quality of the whole gold platting. Due to the mixture and mismatching grain structures of the two gold layers, the duplex gold deposits have less overall porosity compared to the other gold plating types. Moreover, this type of plating allows for more excellent corrosion resistance while consuming less overall gold than single-layer gold deposits.
Steps for Gold Plating
The steps for gold plating an object are as follows:
Preparation: To perform the gold plating, we need to first remove the dirt and oil present on the surface of the object by polishing, tumbling, stripping, sandblasting, and other cleaning procedures, which can be used to further prepare the surface.
Cleansing and Rinsing: In this step, the surface of the object is exposed to electro-cleaning, steaming, or ultrasonic cleaning, and then the piece should be rinsed with water with the aim of removing any remaining residue from the purifying agents. After this stage, the surface is cleaned and can be used for the next step.
Strike Layer: For improving the bonding among plating material and substrate, we add a buffer layer, where nickel is usually used and plated onto the metal. By the way, the object is rinsed again after the addition of the strike layer.
Base Coating: On the base material, additional coating layers are applied. Generally, several layers are added, consisting of copper, nickel, and gold on an individual piece.
Final Coating: The piece is submerged into a plating bath of gold ions, carefully maintained at a particular voltage and temperature. When the process of gold plating starts, the gold is attracted towards the metal and forms a layer over it. Remember that we must use different temperatures and voltages for multiple metals.
Final Rinse: The object will be rinsed using water once again and then left to dry. Make sure that you get the anticipated results. It might be necessary to rearrange and repeat the process if the results obtained through the gold plating process are not satisfactory.
Benefits of Gold Plating

Gold electroplating consists of many benefits, and some of these include:
Electrical Conductivity
Silver and copper being the only ones above gold makes gold the third most conductive metal in the world (21.4 nΩm). However, gold is still considered to be used over the other two in many situations due to it not forming any oxides or compounds and being able to retain its electrical conductivity much better than both copper and silver over long periods and in harsh conditions. That is why stainless steel and copper contacts are commonly gold plated to guarantee a reliable and steady conductive path is formed due to gold’s very specific property.
Thermal Conductivity
Gold is the third most thermally conductive metal at 315 W/mK. Similar to its electrical conductivity, gold gets beaten by silver and copper only. Gold preserves its high conductivity even in extreme conditions due to it not forming any insulating compounds on its surface. This feature allows it to be used in extreme applications such as in outer space and down-hole drilling.
Ductility
Gold is perfect for making flexible contacts or springs as it is highly ductile. Even after constant contact cycles, gold can retain its features due to its high ductility. For your gold springs or plated contacts to ensure the entire finish meets the design requirements, it is necessary for the under-plate selection and application to be critical and have the capability to offer the desired results.
Non-Reactivity
Gold does not react with other elements it comes in contact with or form compounds. The reason is that gold alludes to a noble metal. This property is also the reason why even after millions of years we can still find gold in nature in the form of nuggets. Besides, this feature is also the reason why we use gold as an outer coating on other metals as it provides excellent corrosion resistance.
Corrosion Resistance
Gold even in extreme conditions is considered to be highly resistive to corrosion. Once again, its reason is that gold remains a noble and unreactive metal in nature. Let’s consider some examples. Strong oxidizing and mineral acids are resisted by gold. In addition, it also resists aggressive compounds like hydrogen sulfide and other corrosive salts and brines. Likewise, gold’s engineering properties remain consistent because it cannot form oxides or compounds it comes in contact with. Surprisingly, this characteristic of gold makes it perfect to use as a coating of various exposed components. For instance, it can be used in the manufacturing of battery contacts, interconnect pins, springs, and other similar components, which are openly present in corrosive environments.
Non-Hazardous
As gold does not react with other elements, it is used in the manufacturing of gold-plated medical devices, which can be safely used and put into the human body without worry of it reacting with the body. Gold can endure autoclave cycles exceptionally well and is naturally resistant to germs and bacteria, making it perfect for orthopedic devices. Also, gold appears to be an ideal coating material for marker bands or other arthroscopic tools due to it being radiopaque.
Visual Appeal
Objects, which are electroplated with gold, do not get tarnished or oxidized as long as the coating of the gold is thick enough. The coating of the gold also improves the exterior appearance of the object. The products made from gold are denoted as quality products in any application it is used in since the fundamental value of gold is recognized by the whole universe.
Ending Notes
It is necessary to remember that the gold plating should have a properly engineered finishing to enjoy all the benefits it provides. For an overall successful finish, the under-plates that are usually deposited before the gold topcoat are just as essential to consider as the thickness and type of gold. Additionally, the proper pretreatment and activation of the base metal are critical elements that ensure the highest level of the ultimate gold deposit. What we suggest here is that you must approach a professional plating service provider whose expert team may provide you with the design guidance and a developed test plan for any application or component.

